Abstract
Background: Despite several large studies showing no increased risk for overall venous thromboembolism (VTE) after COVID-19 vaccination, lingering concern remains among some patients and physicians. This may be due to large observational studies that have shown higher rates of VTE after COVID-19 vaccination compared to influenza vaccinations.
Methods: The aim of this study was to compare VTE rates after COVID-19 and influenza vaccinations in a large cohort in the United States with robust multivariable adjustment of comorbidities to exclude residual confounding. Adult COVID-19 vaccinated (Pfizer, Moderna, or Janssen) patients between 11/1/2020 and 11/1/2021 and influenza vaccinated patients between 7/1/2019 and 4/1/2020 were analyzed using electronic medical records. The primary outcome was imaging confirmed acute VTE (upper or lower extremity deep vein thrombosis or pulmonary embolism) occurring within 90-days after the vaccination.
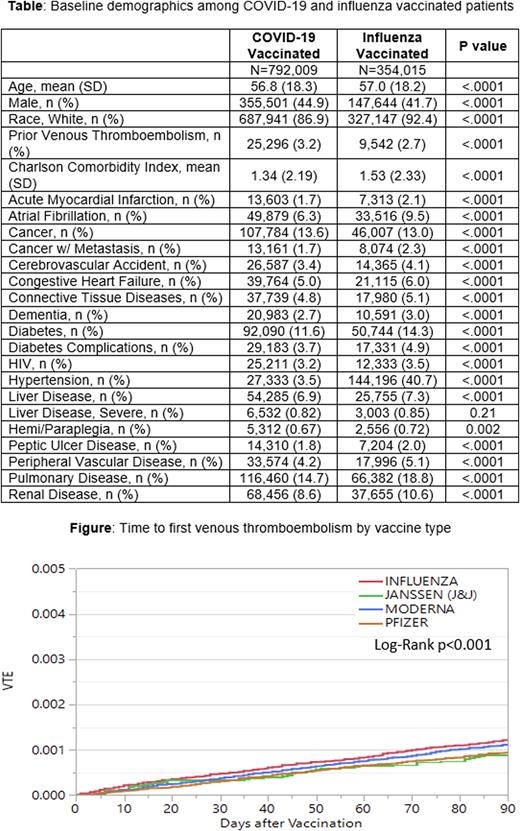
Results: A total of 1,146,024 vaccinations were identified with influenza (N=354,015) or COVID-19 vaccines (n=792,009: Pfizer, n= 452,950, Moderna, n= 290,606, and Janssen (Johnson & Johnson), n= 48,453). The mean age of patients with COVID-19 and influenza vaccinations was numerically similar (56.8 vs 57.0). Most vaccinated patients were female (56.1%), and most were white (88.6%). Small, but statistically significant differences were found in nearly all baseline comorbidities between the two types of vaccines (Table 1). Mean Charlson comorbidity scores were higher in influenza vaccinated patients compared to COVID-19 vaccinated patients (1.53 vs 1.34, p<0.001). In the 90 days after vaccination, a total of 443 VTE events occurred after influenza vaccination and 793 occurred after COVID-19 vaccination (0.13% vs 0.10%, p<0.001). The unadjusted hazard ratio (HR) demonstrated a higher risk of VTE after influenza vaccination when compared to COVID-19 vaccinations (HR 1.22, 95% CI 1.08-1.37). The time to first VTE by type of vaccine is shown in the Figure. After multivariable adjustment (all significant comorbidity differences in Table) there was no statistically significant difference in VTE rates between the COVID-19 and influenza vaccines (aHR 0.97, 95% CI 0.83-1.13).
Conclusion: In this large cohort of COVID-19 vaccinated patients in the United States, the risk of VTE after vaccination after accounting for differences in comorbidities was no different than a pre-COVID-19 historical cohort of influenza vaccinated patients.
Disclosures
Pruthi:Instrumentation Laboratory: Honoraria, Membership on an entity's Board of Directors or advisory committees; HEMA Biologics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy. Sridharan:Alexion Pharmaceuticals: Consultancy. Padmanabhan:Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees; Mayo Clinic, Versiti, Retham Technologies: Patents & Royalties; Retham Technologies: Other: Equity owner and officer.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal